How to stay alive...underwater (Part 1)
Memorial Day and warm weather are finally here. For us, this means a chance to dust off our scuba gear and spend some quality time underwater, logging training dives at our local scuba park in order to prepare for an upcoming GUE Fundies course in July.
There are few activities that we enjoy more than scuba diving, and staying alive is definitely one of them. Thus in order to enjoy our diving we need to be confident in our safety. There are enough factors which contribute to safe diving to fill many many volumes and fuel endless debate, but in this post we will focus on air consumption. To be a little pedantic, the two of us almost never use the term “air” when we dive, since we usually fill our cylinders with enriched air nitrox, so for the rest of the post we will use the neutral term “gas” instead of “air”.
When we are underwater, we constantly monitor the pressure in our cylinders using our gauges. Once the pressure falls to a pre-computed level, we end the dive and ascend to the surface. How exactly do we compute this level for an open water dive? In this post we lay out the ingredients to answer this question, according to a model taught to us by our scuba mentor Bob Sherwood. In our next post we will tie it all together and actually crank through the calculations. Our mathematically inclined readers could do the calculations on their own. Hint: it’s a piecewise linear integral.
A quick note: there are as many ways to approach this question as there are dive training agencies, and there are a hell of a lot of these. Surprisingly, we were taught very little about gas consumption during the many scuba courses we took before we met Bob. Instead, we relied on unreliable divemasters who gave vague instructions such as: “start ascending when you have about 800 psi in your tank or the boat will leave you.” We have learned in retrospect that these statements are simply inappropriate for most of the dives we do. In some cases they are unsafe because they leave too little gas to support a safe ascent, and in other cases they waste perfectly good gas and bottom time by being too conservative. Determining our own gas consumption needs is not a difficult calculation, so we prefer not to rely on the word of a stranger. We now run through these calculations before every single dive, even if we have been to the site before. (We do the math independently, and compare our answers as a safety check. We usually agree, which is good for our marriage).
We speak imperial
One of us is technically an EU citizen, but when in New York he does as the Americans do, and that means expressing volume in cubic feet instead of liters, pressure in pounds per square inch (psi) instead of bar, and depth in feet instead of meters.
Surface air consumption rate
Our surface air consumption (SAC) rate is about 0.75 cubic feet per minute. In other words, when we are hanging out normally above water, we consume about 0.75 cubic feet of gas every minute. Surprisingly, we never knew this our first few years of diving, partially because it was not covered in any of the three courses we took with our PADI instructors. We consider this a shortcoming of their curriculum. Divers must know how quickly they are consuming gas in order to safely plan and execute their dives.
We measured our SAC in four feet of water in a pristine outdoor swimming pool of the nicest days of the year. In other words, there was no surge, no current, and no stress. Under normal scuba conditions which are more challenging than a shallow swimming pool, we generally use a SAC rate of 1.0 cubic feet per minute in our calculations to give us a safety margin.
Ambient pressure
Air pressure at sea level is 14.7 psi, which is also called 1 absolute atmosphere or 1 ata. The ambient pressure in water increases by 1 ata for every 33 feet of depth. We add the air pressure to the water pressure, so diving 33 feet below the surface sums to 2 atas, diving 66 feet below the surface translates to 3 atas, and so forth.
When we’re under water we use scuba regulators to deliver the gas we breathe, which is delivered at the same pressure as the ambient pressure. If they always delivered gas at 1 ata our diaphragm would not be able to overcome water pressure below more than a few feet of water. Thus we would not be able to inhale and scuba would be considerably less fun.
If the ambient pressure is 2 atas, we use twice as much gas with every breath compared to the surface. This means we consume our gas at twice our SAC rate when we are at 33 feet. Similarly, we consumer our gas at three times our SAC rate when we are at 66 feet below the surface, and so forth.
Safety stops and ascent rate
We have already covered that we consume gas underwater much faster than our SAC rate. At this point many people start talking about partial pressures of the gases they breathe, but we won’t go into that here because we don’t need it for this article. Suffice it to say that we inhale and absorb a lot more nitrogen when we are scuba diving than when we are going about our normal lives above water.
The human body is quick to absorb nitrogen yet slow to offgas it through breathing. This unfortunate imbalance is the cause of decompression sickness, commonly called the bends, when a drop in ambient pressure causes nitrogen bubbles to form in the body. If you want to have a graphic idea how horrible this can be, watch this video and imagine that it is you that’s filling with bubbles instead of a block of gelatin. For yet another insight into the damage that decompression illness can do, skip to the end of Bernie Chowdury’s fantastic yet tragic book chronicling how two divers died from explosive decompression after exploring a the famous sunken German U-boat close to New Jersey.
While it is unlikely that we will experience explosive decompression on our shallow dives at Dutch Springs, we still impose a strict speed limit when ascending. Our maximum ascent rate when diving is 30 feet/min at depths below 33 feet, and 10 feet/min at shallow depths between 0 and 33 feet where the gradient of pressure is greater. Furthermore, when ascending from the bottom of our dive, we do a series of one-minute safety stops, spaced 10 feet apart in depth and starting at approximately half our maximum depth. Combined with adequate surface intervals which we compute from dive tables, this reduces the risk of decompression sickness (although it always remains a possibility).
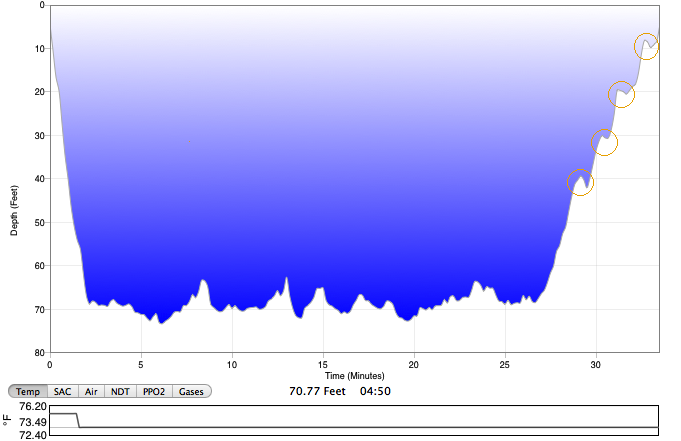
For example, above is a profile of a dive we did off of Pompano Beach last year, logged using our Suunto computers and MacDive software. Maximum depth for this dive was 70 feet, so we took a series of four one- minute safety stops at 40, 30, 20, and 10 feet, circled in orange on the right, with four one- minute ascents in between each stop. We took eight minutes total to ascend from 40 feet to the surface.
We use aluminum cylinders
Although we put steel tanks on our Christmas wish list, we’re still diving with aluminum 80s, the most popular type of cylinder for recreational scuba diving. One slightly shitty thing about them is that, despite their name, they actually only hold 77 cubic feet of air when filled to their to 3000 psi limit.
Your buddy needs to breathe
We adhere strictly to the dive buddy system. This means that when we dive, we keep a close eye on each other in order to assist in the case of an emergency, such as running out of gas or equipment failure. In terms of our gas consumption, this means we need to always have enough gas in either of our cylinders to support a safe ascent for both of us, including safety stops. In other words, if you figure out how much gas you need from the previous two sections of this article, multiply it by two if you want to ensure your buddy returns to the surface with you.
blog comments powered by Disqus